Description
TheraSeed® Pd-103 is indicated for the treatment of localized and unresectable tumors with low to moderate radiosensitivity. Tumors may be recurrent or residual following external beam radiation or excision of primary tumor.
Backed by more than 35 years of clinical use
Unrivaled clinical experience
- Pd-103 shorter half-life and lower energy photons provide distinct radiobiologic advantages over I-125, and may achieve greater disease control over I-1251
- Pd-103 implants demonstrate greater dose heterogeneity, which is associated with improved oncologic outcomes1
- 16.9 day half life; 20-23 keV principal energy
Trusted quality
- Pd-103 produced on our own cyclotrons and seeds manufactured in Buford, GA
- Dosimetric characterization of TheraSeed® Pd-103 include Monte Carlo and experimental studies conducted in accordance with the AAPM Task Group 43 update (TG43U2)2
Exclusive design
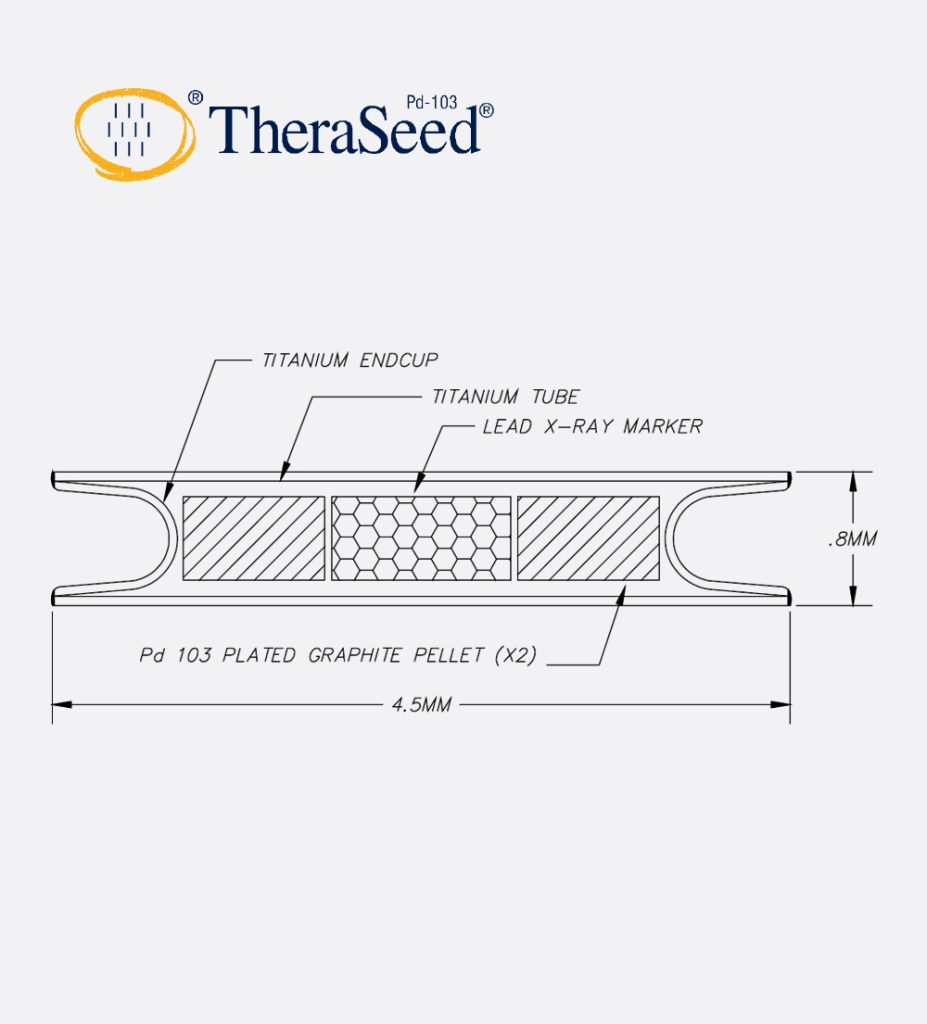
- Distinctive concave end cups designed to facilitate tissue fixity
- Laser welded titanium capsule containing two Pd-103 plated graphite pellets and a lead x-ray marker
- Available in a wide range of activities with both standard and high activity options
Pd-103 shorter half-life and lower energy photons provide distinct radiobiologic advantages over I-125, and may achieve greater disease control over I-125. 1
Pd-103 implants demonstrate greater dose heterogeniety, which is associated with improved oncologic outcomes. 1

Interested in learning more?
To find out how to integrate TheraSeed® into your practice, speak with one of our brachytherapy experts.

- Tang, et. al., “Outcomes after PD-103 versus I-125 for low dose rate prostate brachytherapy monotherapy: an international, multi-institutional study”, Radiotherapy and Oncology, Volume 183, June 2023, 109599.
- Rivard, et al., “Update of AAPM Task Group No. 43 Report: A revised AAPM protocol for brachytherapy dose calculations”, Med Phys. 31, 663-674, (2004)
TheraSeed® Pd-103 source and accessories should be used by physicians who are qualified by training and experience in the safe use and handling of radionuclide brachytherapy sources and whose experience and training has been approved by the appropriate government authorities authorized to license the use of radioactive materials.
Information intended for the USA. Indications may vary in different countries; please either consult the appropriate IFU or your local distributor.


